Tramadol
Tramadol
CAS Number:
27203-92-5
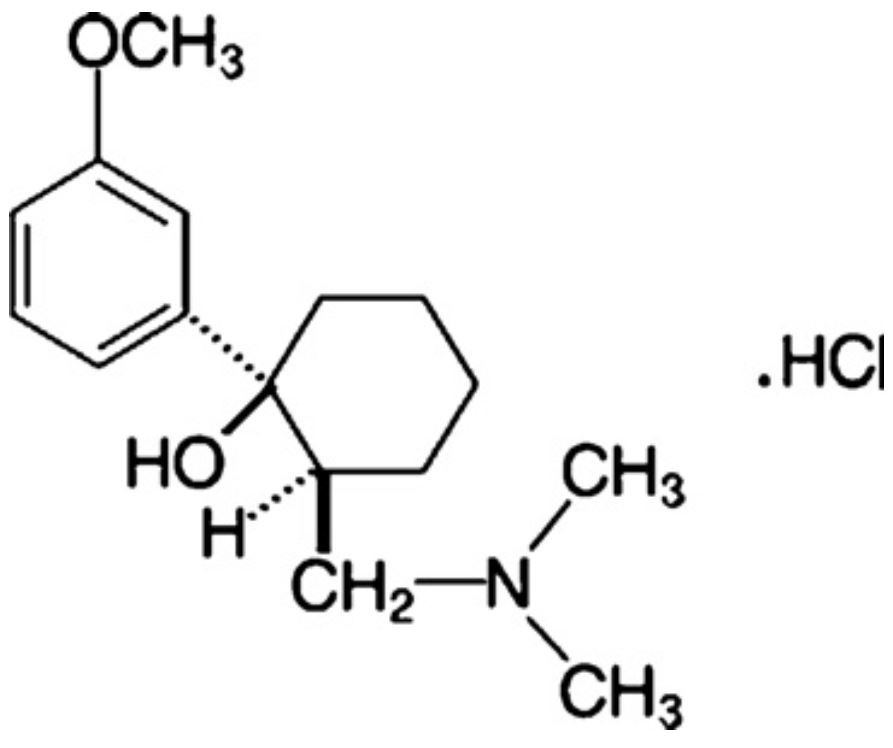
Batch Molecular Formula:
C16H25NO2
Batch Molecular Weight:
263.381 g/mol
Physical Appearance:
White Crystalline Powder
Solubility:
Soluble in Water and Ethanol
Storage:
Store at RT
Tramadol is a centrally acting synthetic painkiller used for moderate to moderately severe pain. It works by activating opioid receptors and blocking serotonin and norepinephrine reuptake, reducing pain signals.
Types of Tramadol
1. Medical Pharmaceutical Formulations
Immediate-Release (IR):
Provides faster pain relief for acute pain.
Duration: 4–6 hours.
Extended-Release (ER):
Designed for long-term pain management (chronic pain).
Duration: 12–24 hours.
Often taken once daily.
2. Combination Products
Tramadol is sometimes combined with other drugs to improve pain control:
Tramadol + Acetaminophen (Paracetamol)
(used for stronger pain relief than either alone)
3. Dosage Forms
Tablets, capsules, Injection and liquid solutions.
Delphis Pharma – Leading Tramadol Manufacturer & Contract Manufacturer in India & Global Markets
Delphis Pharma is a trusted manufacturer and contract manufacturer of Tramadol in India and international markets. We offer custom manufacturing solutions, competitive pricing, and seamless logistics tailored to your needs. Partner with us for high-quality Tramadol production and global supply.
Contact our sales team today for personalized deals and efficient delivery solutions!
Effects
1. Short-term Positive Effects
Pain relief (especially moderate pain).
Mild euphoria or sense of wellbeing (in some people).
Relaxation and sedation.
2. Short-term Negative Effects
Drowsiness, dizziness, and impaired coordination.
Nausea, vomiting, constipation, and dry mouth.
Confusion, sweating, and itching.
Risk of seizures
Risk of serotonin syndrome
3. Long-term Effects
Dependence and addiction.
Tolerance (needing higher doses to get the same effect).
Mood changes and mental health issues (e.g., depression, irritability).
Usage
1. Therapeutic Effects:
Relieves moderate to moderately severe pain by acting on opioid receptors and inhibiting reuptake of serotonin and norepinephrine.
Improves physical functioning and comfort in conditions such as post-operative pain, injury-related pain, or chronic pain (when appropriately prescribed).
2. Side Effects:
Common:
Nausea, vomiting, and constipation.
Drowsiness, dizziness, and fatigue.
Headache, dry mouth, and sweating.
Less Common:
Itching (pruritus) and flushing.
Mood changes (e.g., irritability, anxiety).
Blurred vision or difficulty concentrating.
3. Serious Adverse Effects:
Respiratory depression on higher doses.
Seizures on higher doses.
Disclaimer
Products falling in to controlled substance category / list of schedule drugs shall be exported directly from the manufacturer principals only against receipt of original import permission issued by concerned authorities of the importing country. Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer’s risk. Most OTC drugs are not reviewed and approved by FDA, however, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. This is only for study not for any Human / Veterinary use. (Condition Apply)* Delphis Pharmaceutical India warns the buyers / importers not to use/sell/procure any goods to achieve a product which might be knowingly causing an infringement / controlled / banned drugs. Delphis Pharmaceutical India is not responsible or liable for the same.