![]()
API
![]()
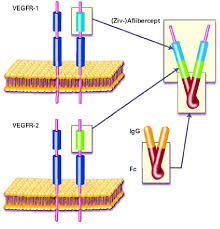
Aflibercept Eylea
CAS NO: 862111-32-8
Batch Molecular Formula
C4318H6788N1164O1304S3
Batch Molecular Weight
96.90
Storage
Store at RT
Batch Molecular Structure
MEDICAL USE:
- Aflibercept is used to treat certain serious eye conditions (such as wet age-relatedmacular degeneration – wet AMD, macular edema following central retinal vein occlusion, diabetic macular edema). This medication can help preserve vision and prevent blindness.
Disclaimer
- Products falling in to controlled substance category / list of schedule drugs shall be exported directly from the manufacturer principals only against receipt of original import permission issued by concerned authorities of the importing country. Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer’s risk. Most OTC drugs are not reviewed and approved by FDA, however, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. This is only for study not for any Human / Veterinary use. (Condition Apply)* Delphis Pharmaceutical India warns the buyers / importers not to use/sell/procure any goods to achieve a product which might be knowingly causing an infringement / controlled / banned drugs. Delphis Pharmaceutical India is not responsible or liable for the same.