![]()
API
![]()
Abacavir (ABC)
CAS NO: 136470-78-5
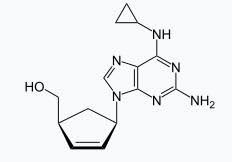
Batch Molecular Formula
C14H18N6O
Batch Molecular Weight
286.332 g/mol
Physical Appearance
White to off white Solid
Melting point
165 °C (329 °F)
Storage
Store at RT
Batch Molecular Structure
MEDICAL USE:
HIV DRUG – nucleoside reverse transcriptase inhibitors.
- Abacavir tablets and oral solution, in combination with other antiretroviral agents, are indicated for the treatment of HIV-1 infection.
- Abacavir should always be used in combination with other antiretroviral agents. Abacavir should not be added as a single agent when antiretroviral regimens are changed due to loss of virologic response.
Disclaimer
- Products falling in to controlled substance category / list of schedule drugs shall be exported directly from the manufacturer principals only against receipt of original import permission issued by concerned authorities of the importing country. Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer’s risk. Most OTC drugs are not reviewed and approved by FDA, however, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. This is only for study not for any Human / Veterinary use. (Condition Apply)* Delphis Pharmaceutical India warns the buyers / importers not to use/sell/procure any goods to achieve a product which might be knowingly causing an infringement / controlled / banned drugs. Delphis Pharmaceutical India is not responsible or liable for the same.